
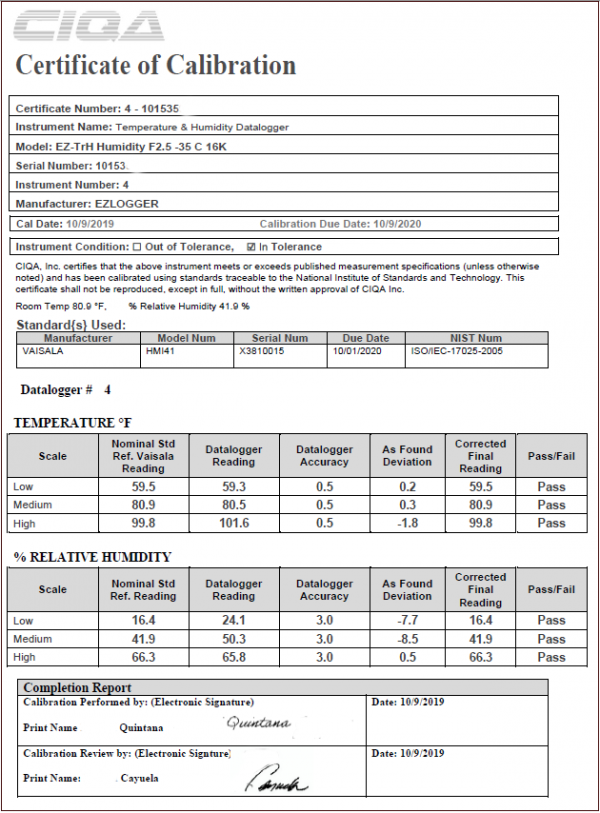
Linear regression model in which the differences between observed and predicted values (residuals) have been shown. There are international guidelines for the validation of the analytical methods, which need to be followed closely in order to have more consistent data throughout different laboratories and increase the chance of their acceptability by the regulatory authorities.
This will be achieved during validation of the analytical method.
In the following sections, each of those parameters is explained, and few practical examples have been used to further discuss the concepts.Īfter the calibration model is chosen, it is required to demonstrate that all future measurements will be close to the true values of the content of the analyte in the sample. Other characteristics of the calibration curve, including regression model, slope of the line, weighting and correlation coefficient, need to be carefully evaluated. A linear calibration curve is a positive indication of assay performance in a validated analytical range. The quality of a bioanalytical method is highly dependent on the linearity of the calibration curve. However, the main concepts are applicable to the other analytical approaches. This chapter is more focused on the bioanalytical methods in which an analyte is measured in blood, plasma, urine or other biological matrices. The unknown samples can be from a wide range of sources: food and agricultural, pharmaceutical formulations, forensic and the clinical pharmacology studies. This relationship is built to predict the unknown concentrations of the analyte in a complicated matrix. Despite the highly regulated area, some challenges still exist regarding the validation of some analytical methods including methods when no analyte-free matrix is available.Ĭalibration curve in bioanalytical method is a linear relationship between concentration (independent variable) and response (dependent variable) using a least squares method. Most of the international guidelines require that the parameters, including linearity, specificity, selectivity, accuracy, precision, lower limit of quantification (LLOQ), matrix effect and stability, be assessed during validation. After the best regression model is selected, the analytical method needs to be validated using quality control (QC) samples prepared and stored in the same temperature as intended for the study samples. Using an internal standard corrects for the loss of analyte during sample preparation and analysis provided that it is selected appropriately.
Some statistical analyses are required to choose the best model fitting to the experimental data and also evaluate the linearity and homoscedasticity of the calibration curve. Calibration curve is a regression model used to predict the unknown concentrations of analytes of interest based on the response of the instrument to the known standards.


 0 kommentar(er)
0 kommentar(er)
